Navigating the Regulatory Maze: Essential Skills for a Successful Career in Drug Regulatory Affairs
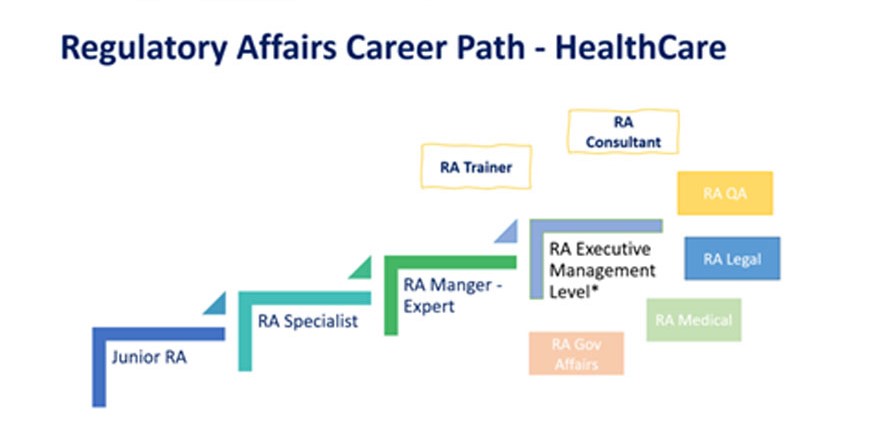
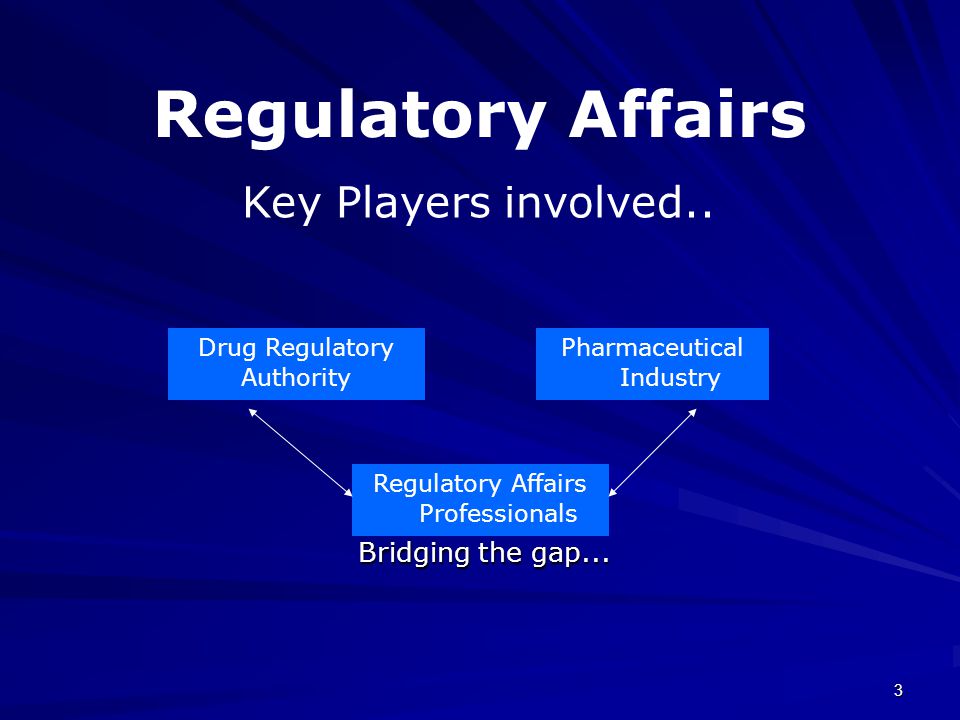
Introduction Brief overview of drug regulatory affairs and its importance in the pharmaceutical industry. Drug regulatory affairs is a vital component of the pharmaceutical industry, ensuring that drugs meet all the legal and regulatory requirements for development, approval, and distribution. This field involves overseeing the complex processes of drug research, clinical trials, regulatory submissions, and […]