Online Certificate Courses in Drug Regulatory Affairs
Drug Regulatory Affairs plays a vital role in balancing the interests of Pharmaceutical companies, healthcare providers, and patients by promoting the development and use of safe and effective drugs. It is a critical component of the healthcare system that contributes to public health and patient safety. Regulatory agencies in different countries, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are responsible for overseeing and implementing drug regulation. Skillbee Solution has brought an opportunity to become a Certified Drug Regulatory Affairs Professional for those who understand the basic stages of the industrial production life cycle and their regulatory requirements. Online Certificate Courses in Drug Regulatory Affairs would impart among the candidates the ability to examine specific regulations that governs the Pharma industry in USA, Europe, UK, Canada, Japan, India etc. The curriculum of the certification is designed as a comparative analysis of Pharma regulatory systems of different nations integrated with concrete management tools of the supply chain like, Certification schemes, Regulatory compliance with government guidelines, product approval procedures etc.
To obtain the certification, the candidate must clear the qualifying exam for which proper training materials will be provided by the our institute for Certificate Course in Drug Regulatory Affairs. No extra charges will be applied for this material.
Our training for the preparation of this certification is aimed at improving the conceptual knowledge of the participant towards formation of efficient public policies, compliance with regulatory guidelines, the regulatory strategies of companies in the food industry, and resolving any dilemmas that a working professional may face at respective workplace. Successful performance in this exam implies that the participant has in-depth knowledge and clear understanding of industry guidelines and regulations. The study resources have been
carefully designed to introduce the participant to various aspects and basics of industrial applications, its need, and benefits in assuring quality production.
The case study based approach in the training provided for the certification program is designed for working professionals in full time employment who want to update their knowledge and gain required skills and attitude in the area in order to become a certified
Drug Regulatory Affairs Professional in the domain. An advanced training having rigorous case studies based methodology will be imparted all participants to prepare for the Certifying Examination We provide Training and Certification for Drug Regulatory Affairs at UK, USA, Benglore and Hyderabad.
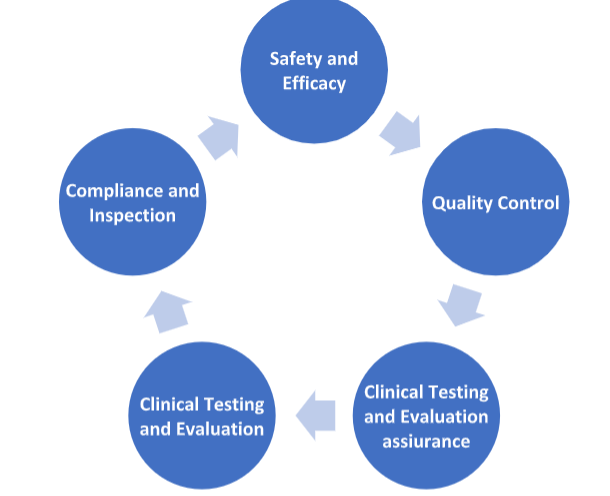
The purpose of drug regulation is to ensure the safety, efficacy, quality, and proper use of pharmaceutical products for the benefit of public health. This regulation is essential to protect consumers, patients, and healthcare providers from potential risks associated with the use of drugs and to promote the development and availability of effective and safe medications. Here are the key purposes and objectives of drug regulation:-
Learning Center :- Distance education is defined as using non-traditional approaches to the delivery of instruction for the purposes of education.
Education Center: – An education center is a place or institution where students get knowledge or attend classes.
Educational Consultant: – An education consultant is often someone with teaching or administrative experience now serving as an advisor in all education.
Training Center and Educational institution: – A university engages in broad education while a training center engages in training of certain people which is narrow, specific, organization-oriented and short-term.
Tutoring Service: – Tutors help students learn the material in individual courses while coaches help students learn how to be successful in their career.
Study Modules
- Regulatory Authorities around the world
- Various stages in Drug development
- Introduction to the GMP concept
- Methodological regulatory filing around the world
- Common Technical Document Filing. eCTD submission process
- Intellectual Property Rights in Pharma Industry
- Quality Assurance and Drug Regulations, ICH and WHO guidelines
- Guidelines for Import and Export of Pharma products
- Audits by the Government and Regulatory bodies
- Breach reports and Compliance guidelines
- Drug Registration in African Countries.
- Drug Registration in Gulf (GCC) Countries.
- AYUSH Regulatory Affairs (Ayurveda, Unani, Siddha, Homeopathy, Yoga and Naturopathy)
- Industry specific case studies
Eligibility
Graduation/ B.tech/ B.Sc. in Microbiology/ Life Sciences/ Botany/ Zoology/ Food Science/ Food Technology/ BE/ B.Pharma/ MBBS/ BDS/ BHS/ BUMS/ BAMS or any other discipline. Diploma holders are eligible for our Executive Diploma, Industry Certificate, and Certificate Programmes.
Upon Successful completion of Course our team will provide Following
- Resume Preparation
- Interview Preparation and Mock Interview Session.
- Communication skills and personality development sessions
- Placement Assistance
Course Registration
The registration will be carried out by contacting on Skillbee Solution. How to Apply
Program Deliverables
A comprehensive study material for all the modules in Soft copies ensuring the needs of the audience. The accompanying training material is appropriately aligned with the current Industry’s expectations.
Assignments for all the program modules for continuous evaluation and guidance.
Interactive or online live sessions on all key areas of the program giving all flexibility to the participants.
Online classes for all the modules will be conducted on the weekdays or Weekend (as per batch you choose). Moreover, a doubt clearing session will also be scheduled before the examination.
All the efforts are made by faculty members to make the entire program modules easily understandable.
Assessment and evaluation for all the program modules in order to enhance the levels of competencies and skills of the participants leading towards the objective of application in the job.
At the end of each program modules, the trainers shall obtain feedback from the participants using specially designed questionnaires.
All learning and training delivery initiatives shall be conducted in English.
Certification and Membership
Contact on +91-8103635949, +91-9691633901 or Info@skillbee.co.in for details of fees structure
Assessment & Cetification
All the participants are expected to appear for online assessment. After successfully qualifying the examination, the participants will be certified as GCP Professional by Company Connect
Placement Assistance & Corporate Relations
The Institute has partnered with many organizations for providing Drug Regulatory Affairs Training and Placement to in its participants. Besides, it has a robust placement cell comprised of senior level Human Resources professionals and Talent Acquisition experts which
maintains close links with business and Industry. In recent months the Institute has witnessed more and more participation
from professionals working with global pharmaceutical, healthcare and food giants like IQVIA, Dr. Reddy’s Laboratories, Aurobindo Pharma, Glenmark Generics, Cipla, Wockhardt, Pfizer, Abbott, Medtronic, Foster Corporation, IPCA Laboratories, Calyx,
Mother Dairy, Bliss GVS Pharma, Al Rawabi, Almarai, Green Pastures, SeQuent, PepsiCo India, Mankind, Beryl Drugs, Allergy Therapeutics, CFTRI, Ciron, Sun Pharmaceutical, Novartis, GlaxoSmithKline, Ranbaxy , Biocon etc.
