What Are URS, FS, DS, IQ, OQ, and PQ in Computer System Validation (CSV)?
In Computer System Validation (CSV), you will often hear terms like URS, FS, DS, IQ, OQ, and PQ. For beginners, these can sound confusing and very technical. But in reality, they are just steps that help ensure a system works correctly and meets its intended purpose. These documents and activities are part of the validation lifecycle. […]
Will AI Replace Computer System Validation (CSV), or Make It Stronger?
What is the Real Purpose of CSV ? Computer System Validation is not just about testing software. Its main purpose is to make sure that a computerized system works correctly, produces reliable results, and follows regulatory requirements. In industries like pharmaceuticals, medical devices, and laboratories, validated systems help protect patient safety and ensure data accuracy. CSV […]
Best Training Institute for CSV – Your Complete Guide to Building a Successful Career in Computerized System Validation
Introduction: Why CSV Is One of the Most Demanded Careers in Pharma The pharmaceutical industry is evolving rapidly, and with digital transformation becoming the backbone of regulated manufacturing, Computerized System Validation (CSV) has emerged as one of the most in-demand career paths in pharma and life sciences. Today, almost every pharmaceutical company operates through advanced […]
Computer Software Assurance (CSA)V/S Computer System Validation — And Why CSV Still Matters More
As digital systems continue to evolve, regulated industries such as pharmaceuticals, medical devices, and life sciences are rethinking how they ensure software quality and compliance. Traditionally, Computer System Validation (CSV) has been the backbone of compliance. More recently, regulators like the FDA have introduced odern, risk-based alternative. Computer System Validation (CSV) Computer System Assurance Computer […]
Data Migration Using CSV: A Structured and Risk-Based Approach

Introduction In today’s digital world, data migration is a regular part of system upgrades. CSV files are often the first choice because they are simple, lightweight, and work with almost every tool. But moving data using CSV still needs proper planning and validation, otherwise small mistakes can turn into big problems later. HOW DATA MIGRATION […]
“Unified Automation Framework for CSV Verification Management Across Industry-Standard ALM Tools”
Managing Computer System Validation (CSV) verification across multiple Application Lifecycle Management (ALM) tools presents a significant challenge for many industries. Each ALM tool often has its own processes, data formats, and reporting standards, which can lead to inefficiencies, errors, and delays. A unified automation framework designed for CSV verification management can bridge these gaps, providing […]
The Importance of CSV in the Pharmaceutical and IT Industries
Computerized System Validation (CSV) is a critical requirement for ensuring the reliability, safety, and regulatory compliance of software systems used across the pharmaceutical and IT industries. These sectors depend heavily on computerized systems to manage sensitive data, control manufacturing operations, and support regulatory obligations. Without proper validation, system failures can lead to data integrity issues, […]
The Role of LIMS in Ensuring Regulatory Compliance
Introduction In today’s highly regulated laboratory environment, maintaining regulatory compliance is essential for ensuring data accuracy, operational integrity, and public trust. Laboratories operating in industries such as pharmaceuticals, biotechnology, clinical diagnostics, food safety, and environmental testing must comply with stringent regulations and standards. A Laboratory Information Management System (LIMS) plays a critical role in helping […]
Why ER/ES Is Required in Computer System Validation (CSV)
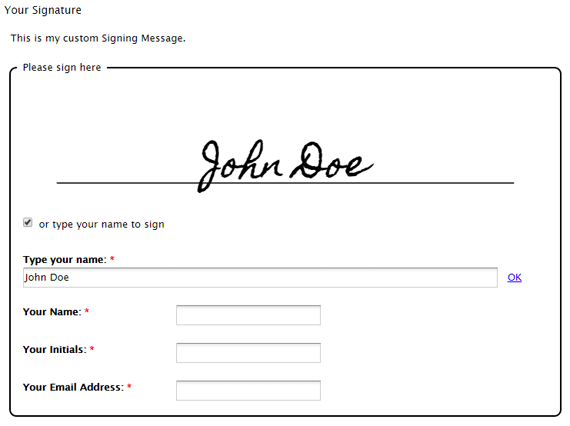
Computer System Validation (CSV) plays a critical role in regulated industries such as pharmaceuticals, biotechnology, and medical devices. Ensuring that computer systems perform as intended and comply with regulatory requirements is essential for product quality and patient safety. Within this process, Electronic Records (ER) and Electronic Signatures (ES) have become indispensable components. This post explores […]
How Automation and AI Are Transforming CSV: Tools, Use Cases, and Best Practices

Working with large CSV files is something almost every industry deals with—whether it’s sales reports, customer records, logs, or system exports. While CSV files are simple by design, managing them at scale can quickly become frustrating. Manual processing takes time, invites errors, and often slows teams down. This is where automation and artificial intelligence (AI) […]
